Anybody, at any moment, can come up with a medical product idea. There are others who can help a revolutionary technology reach the market besides seasoned engineers from big corporations and well-known doctors. You may come up with an idea as you wait for your appointment with the doctor, or perhaps you’re caring for a sick family member and want to get them something to speed up their recovery. The concept frequently ends there for those of us who don’t work for big businesses since the medical device sector is perceived as being overly regulated and replete with obstacles. Many people who have made the decision to take the leap are left wondering how to get started. To learn more about what it takes to make a complete medical product, keep reading.
Idea
Everything begins with an idea. Before moving on to the questions such as how the medical pick and pack process will work, you need to have a precise problem you want to tackle. Like in other industries, medical product idea development also begins with evaluating and identifying the market, determining if there is an unmet demand in that market or whether there is a more effective approach to meet that specific need. Any need that provides a solution, such as a new or improved method of monitoring health, solutions that improve the delivery of healthcare, tools or technology that facilitate better administration, or anything else that promotes health and human life (like vitamins and analgesics), might fall under this category.
Concept
Once an idea has been chosen, researchers will create “proof of concept” materials. The goal of this paper is to show the practicality of a concept by pointing out any logistical or technological problems that might compromise the effectiveness of the product. Many ideas won’t make it through this point, but those that are workable will go on to the latter stages of development.
Prototype
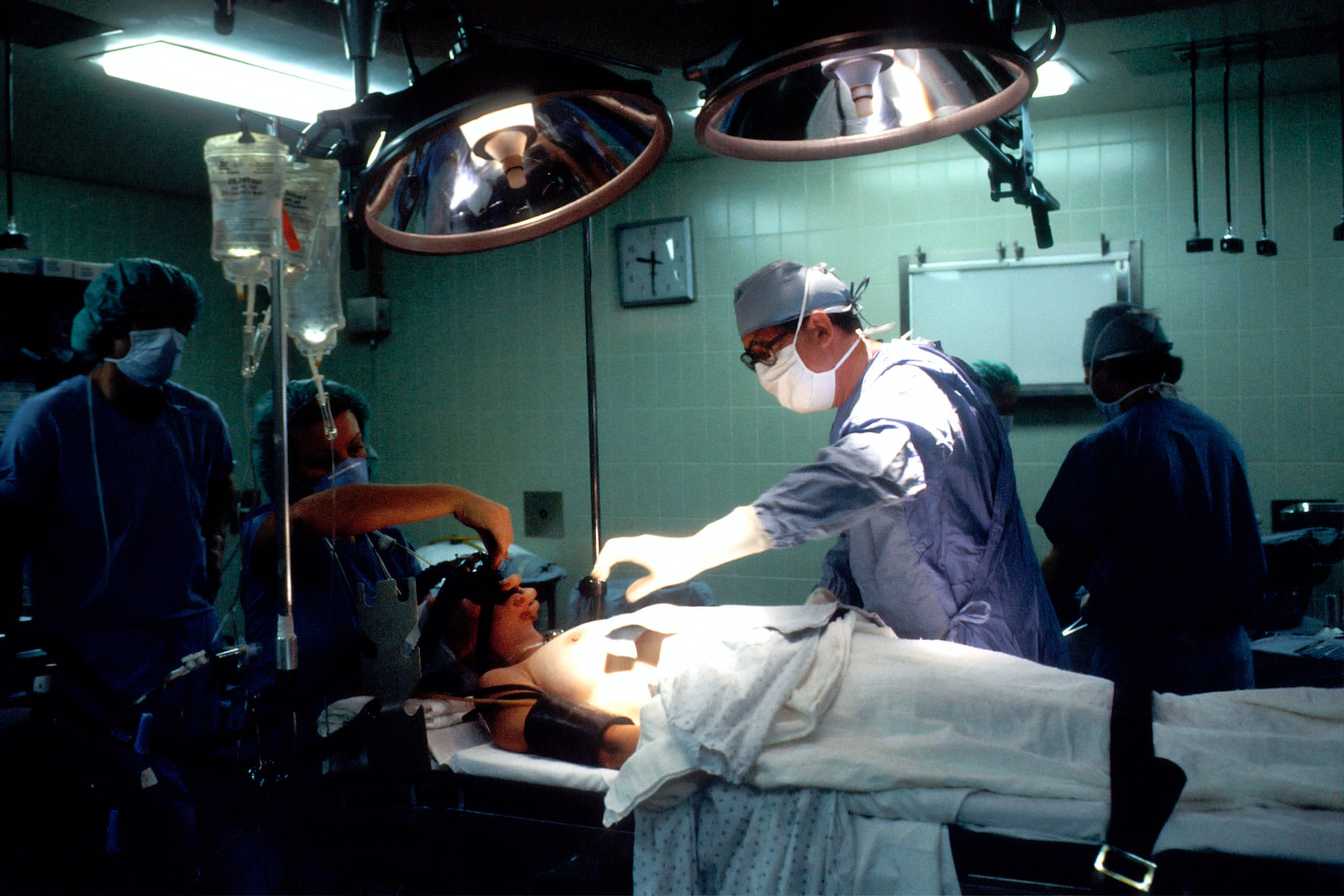
Next step – researchers and engineers construct a prototype medical product. The prototype of the device is not yet fit for human usage. The user and usage environment of any product must be taken into account while it is being developed. This refers to determining if there will be a single user or a number of users of the medical product (doctors, patients, technicians, etc.). What abilities are required for these consumers to use the product effectively?
When it comes to creating and deploying a product in a hospital or healthcare context, product developers have a lot to think about. For instance, it is crucial that we comprehend the sights and sounds of the surrounding environment if we are developing a device that will be utilized in an operating room. We need to know what other noises are available in the same area if we are creating a product that informs consumers using an audio cue. Are there any sounds that will be comparable to our product in pitch and duration? Knowing our users’ demands and the usage environment are essential steps in this phase, and they shouldn’t be left until the conclusion of the product design process.
Fulfilled Regulation and Compliance Needs
Every medical product must go through this procedure. As a result of varying regulations and standards in the EU, the USA, the UK, and other places, the precise product development process for medical devices varies from country to country. The nation’s appropriate regulatory authority is in charge of enforcing these particular regulatory standards. There are some universal processes in the medical device product development lifecycle that are followed, but medical device development should be tailored to the needs of the country in which the device is to be utilized.
Manufacturing
Manufacturing of medical devices encompasses all stages of product creation, from developing a manufacturing process to scaling it up to continual process improvements, perhaps working with someone like BSA Mouldings Injection Moulding Company to help you handle any increased demand. Additionally, it covers sterilizing and preparing a product for shipping. The manufacturing procedure for a medical device may depend on its design but will typically include techniques like 3D printing, additive manufacturing, and laser manufacturing.
Quality Control
Consistency in quality is essential throughout the production process to guarantee that every product fulfills the necessary requirements. To ensure that products meet the required standards, quality control inspects them. Prior to reaching the patient, faulty items must be found.
Deep-level involvement is necessary for any marketable medical product owing to the complexity of the requirements, usage patterns, user experience, laws, associated iterative processes, technologies, materials, and many other factors. You might need assistance from knowledgeable medical product advisors to stay competitive or shorten your time to market, but hopefully, this article covered the essentials for you.